Impurities in Water
- The solubility of the minerals
- Temperature and pressure
- The length of contact
Hardness of Water
The hardness of water is a measure of the calcium and magnesium ions in the water. The ions are components of dissolved salts such as carbonates, sulfates, chlorides, and nitrates. The calcium salts are about twice as abundant as the magnesium salts.
The standard domestic measurement of hardness is grains per gallon (GPG). One grain per gallon is equivalent to approximately 17.4 parts per million (ppm). 7,000 grains equal a pound. Approximately, 1/4 ounce of mineral in 1,900 gallons equals one part per million.
The standard domestic measurement of hardness is grains per gallon (GPG). One grain per gallon is equivalent to approximately 17.4 parts per million (ppm). 7,000 grains equal a pound. Approximately, 1/4 ounce of mineral in 1,900 gallons equals one part per million.
The hardness of water varies greatly depending on the water supply. The range from slightly under 1/2 GPG to over 100 GPG covers practically all natural fresh water supplies. Water hardness is classified as follows:
- 0 to 1.0 GPG - soft water
- 1.1 to 3.5 GPG - slightly hard water
- 3.6 to 7.0 GPG - moderately hard water
- 7.1 to 10.5 GPG - hard water
- 10.6 or more GPG - extremely hard water
Water with greater than 1 GPG has the potential to deposit scales on fixtures, in pipes, and in water equipment such as hot water heaters.
Hard Water Treatment
Hard water is usually treated with the help of an ion exchange process, commonly known as water softening. Softening by ion exchange uses resin beads that, in effect, bind the hardness-causing ions within its matrix.
A sodium chloride or potassium chloride brine solution is used to regenerate the resin when its capacity to remove hardness is diminished. The brine knocks the hardness ions off the resin, so it can be flushed away.
Iron in Your Water Supply
Iron is one of the most troublesome minerals encountered in domestic and commercial water supplies. The various forms of iron can leave stains and deposits that range in color from yellow to brown, but commonly have a "rusty" color. As an organic residue, it may be gray to black, and slimy to rock solid. Iron can also impart undesirable odors and tastes to water.
Iron occurs in many forms in natural water supplies. The most common forms are described below:
Dissolved Iron
Ferrous bicarbonate [Fe(HCO3)2] is found only in oxygen-free water. Dissolved iron is measured in parts per million (ppm). One ppm is equivalent to approximately 1/4 ounce of iron in 1,900 gallons of water. The recommended limit of iron in drinking water is 0.3 ppm and will begin staining at 0.5 ppm. The water containing it is clear and colorless when drawn. Upon contact with the air, oxygen is absorbed and reacts with the dissolved iron to form insoluble ferric hydroxide (commonly known as rust). This clouds the water and colors it in shades of yellow to red-brown.
This reaction produces carbon dioxide as follows:
2Fe(HCO3)2 + 1/2O2 + H2O = Fe(OH)3 + 4CO2
The dissolution of the above carbon dioxide in water also forms carbonic acid. The presence of carbonic acid lowers the pH, and results in low alkalinity of water (2-3 gpg total solids). This can cause some corrosion problems in the system. Filtration through a neutralization media is then required to remediate this. The neutralization media commonly used is composed of calcium carbonate such as limestone, or marble.
Small quantities (less than 0.5 ppm) of ferrous iron, in the absence of dissolved oxygen, may be removed by ion exchange water conditioning. If this method is used, a chemical resin cleaner is required to be added to the brine. This will help remove the exchanged iron and prevent any oxidized iron from fouling the resin bed.
Ferrous iron, in higher concentrations, is most commonly removed by a two-step oxidation then filtration process. Chlorine, oxygen, and potassium permanganate are the most common oxidizers used. The filter media is most commonly manganese greensand.
Insoluble Iron
Insoluble ferric hydroxide in water produces a red water condition. It is found in some natural waters as a suspension of fine, denser-than-water particles. More often ferric hydroxide, in water, is formed when exposed to oxidizing conditions, like percolating from a spring, or being exposed to air at a water tap.
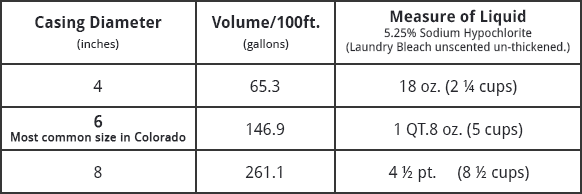
Another source of insoluble iron is from the action of bacteria. Bacteria can produce ferric hydroxide or iron carbonate as a waste product. Chlorine fed into the well or before the pressure tank will control this type of bacterial nuisance.
Manganese and hydrogen sulfide compounds are also found in ground water. Due to their chemical similarities to the above discussed iron compounds, they can be treated and removed from the water using the same methods described above.
Quick Links
CONTACT information
BOULDER / DENVER
Phone: 303-442-1911
NORTHERN COLORADO
Phone: 970-484-6006
Email: john@waterwell.cc